Our Research

SynCat Researchers have much freedom to select and define their own projects. Over the years, our principal investigators worked on topics such as
- Model Catalysts for the Fischer-Tropsch Synthesis (FTS),
- Photocatalysis,
- Oxygen Evolution in Water Electrolysis, and
- Surface Science studies.
Below, we list selection of publications that appeared from these projects. Click here to learn about highlights as presented by individual researchers.
Selected Publications
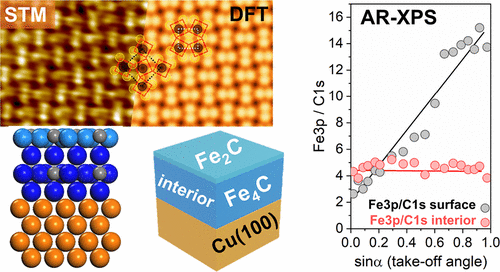
“Atomistic Understanding of the Formation, Structure, and Decomposition of an Fe4C Iron Carbide Phase on a Copper Substrate“
R. Gubo, Pengju Ren, Xin Yu, Tianfu Zhang, Xiaodong Wen, Yong Yang, Yong-Wang Li, J.W. (Hans) Niemantsverdriet, C.J. (Kees-Jan) Weststrate, J. Phys. Chem. C 127 (2023) 12811–12820
Abstract
Having an atomistic understanding of the formation and structural characteristics of bulk and surface iron carbide phases plays a crucial role in comprehending the mechanisms of phase transformation, microstructure formation, and surface reconstructions in the development of advanced materials with improved magnetic, mechanical, and catalytic properties. This study uses synchrotron (SR) and angle-resolved (AR) XPS, STM, LEED, and theoretical calculations (DFT) with the aim to provide a detailed experimental and theoretical discussion on the formation, stabilization, structure, and decomposition of Fe4C iron carbide. FCC(100) Fe4C iron carbide multilayer films were prepared on Cu(100) by evaporating iron in the presence of an ethylene atmosphere. Angle-resolved XPS measurements reveal that surface and interior carbon can be distinguished due to their appearance at different binding energies and reveal that the surface consists of Fe2C while the bulk has Fe4C stoichiometry. LEED and STM measurements show that the surface exhibits the p4g(2 × 2) clock reconstruction. Theory simulations show that the bulk Fe4C iron carbide has a constrained crystal lattice with alternating Fe and Fe4C2 layers, which grow in a pseudomorphic way on the copper. The carbide phase is stable up to 700 K. Above this temperature, iron diffuses into the copper while carbon remains on the surface, where it forms graphite.
An addtion to this article has been published with improved DFT simulations of the Fe4C phase.
Grapical abstracts on this page are copyright protected
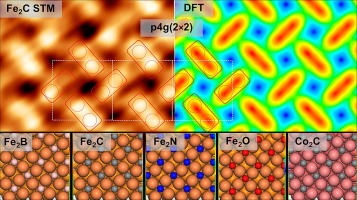
“Similarities and trends in adsorbate induced reconstruction – Structure and stability of FCC iron and cobalt surface carbides“
R. Gubo, Pengju Ren, Xin Yu, Tianfu Zhang, Xiaodong Wen, Yong Yang, Yong-Wang Li, J.W. (Hans) Niemantsverdriet, C.J. (Kees-Jan) Weststrate, Appl. Surf. Sci. 626 (2023) 157245
Abstract
Thin FCC (1 0 0) iron and cobalt carbide films were prepared on Cu(1 0 0) to study the connection between their structure, electronic properties and stability. We present the first detailed, real space experimental confirmation of the C-induced clock reconstruction on the FCC(1 0 0) surfaces of iron and cobalt. Both Fe and Co surface carbides show p4g (2 × 2) surface reconstruction with tetracoordinated square planar carbon and pure FCC (1 0 0) metal layers underneath. Combining tip-sample distance dependent STM imaging with theoretical calculations we present different imaging modes of Fe2C. Using a combination of angle-resolved x-ray photoelectron spectroscopy (AR-XPS), Auger electron spectroscopy (AES), low energy electron diffraction (LEED), scanning tunneling microscopy (STM), and theoretical calculations we provide detailed electronic and structural models for Fe2C and Co2C p4g (2 × 2) surface carbides and other 2D Fe2X interstitial compound systems. In various Fe2X (X = B, C, N, O) surface compounds moving to the right in the periodic table with increasing electrons the reconstruction becomes less favorable, while iron carbide shows the highest stability.
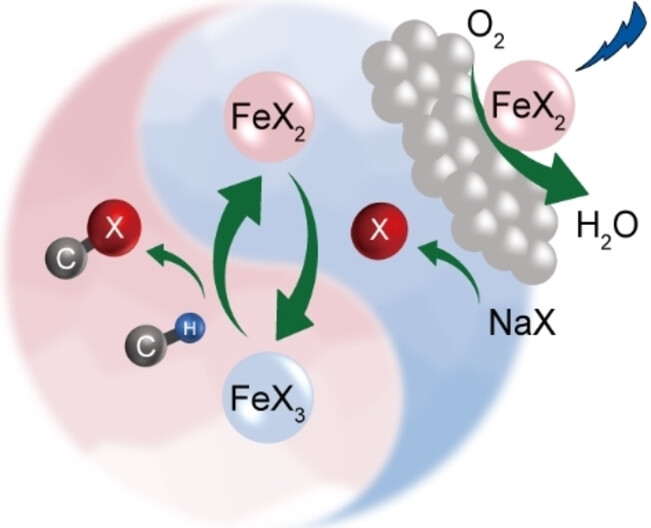
“Heterogeneous Photocatalytic Recycling of FeX2/FeX3 for Efficient Halogenation of C−H Bonds Using NaX”
Jiani Ye, Dongsheng Zhang, S. Salli, Yajiao Li, Feiyu Han, Yuanqiang Mai, F. Rosei, Yongwang Li, Yong Yang, F. Besenbacher, J.W. Niemantsverdriet, Ren Su, Angew. Chem. Int. Ed. 62 (2023) e202302994
Abstract
Environmental-friendly halogenation of C−H bonds using abundant, non-toxic halogen salts is in high demand in various chemical industries, yet the efficiency and selectivity of laboratory available protocols are far behind the conventional photolytic halogenation process which uses hazardous halogen sources. Here we report an FeX2 (X=Br, Cl) coupled semiconductor system for efficient, selective, and continuous photocatalytic halogenation using NaX as halogen source under mild conditions. Herein, FeX2 catalyzes the reduction of molecular oxygen and the consumption of generated oxygen radicals, thus boosting the generation of halogen radicals and elemental halogen for direct halogenation and indirect halogenation via the formation of FeX3. Recycling of FeX2 and FeX3 during the photocatalytic process enables the halogenation of a wide range of hydrocarbons in a continuous flow, rendering it a promising method for applications.
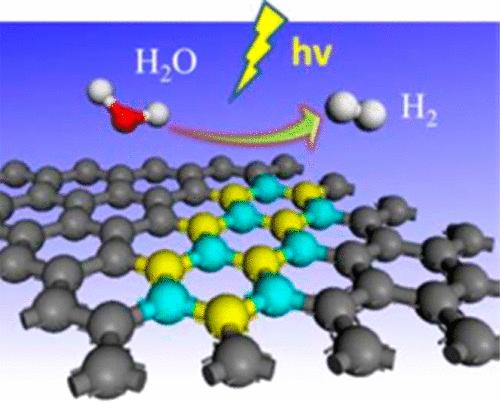
“Photocatalytic Abstraction of Hydrogen Atoms from Water Using Hydroxylated Graphitic Carbon Nitride for Hydrogenative Coupling Reactions”
Dongsheng Zhang, Pengju Ren, Wuwen Liu, Yaru Li, S. Salli, Feiyu Han, Wei Qiao, Yu Liu, Yingzhu Fan, Yi Cui, Yanbin Shen, E. Richards, Xiaodong Wen, M.H. Rummeli, Yongwang Li, F. Besenbacher, J.W. Niemantsverdriet, Tingbin Lim, Ren Su, Angew. Chem. Int. Ed. 61 (2022) e202205356
Abstract
Employing pure water, the ultimate green source of hydrogen donor to initiate chemical reactions that involve a hydrogen atom transfer (HAT) step is fascinating but challenging due to its large H−O bond dissociation energy (BDEH-O=5.1 eV). Many approaches have been explored to stimulate water for hydrogenative reactions, but the efficiency and productivity still require significant enhancement. Here, we show that the surface hydroxylated graphitic carbon nitride (gCN−OH) only requires 2.25 eV to activate H−O bonds in water, enabling abstraction of hydrogen atoms via dehydrogenation of pure water into hydrogen peroxide under visible light irradiation. The gCN−OH presents a stable catalytic performance for hydrogenative N−N coupling, pinacol-type coupling and dehalogenative C−C coupling, all with high yield and efficiency, even under solar radiation, featuring extensive impacts in using renewable energy for a cleaner process in dye, electronic, and pharmaceutical industries.
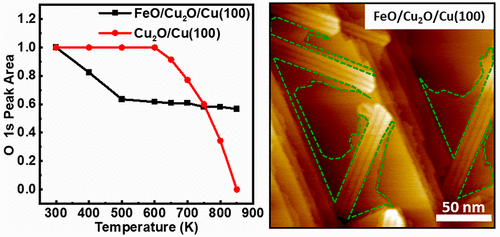
“Role of Interfaces in the Thermal Reduction Process of the FeO/Cu2O/Cu(100) Surface”
Guihang Li, Xin Yu, C. J. Weststrate, Pengju Ren, Jian Xu, Qian Xu, Yong Yang, Yongwang Li, J. W. Niemantsverdriet, Xiaodong Wen, and Junfa Zhu, J. Phys. Chem. C 125 (2021) 20863–20869
Abstract
FeO(111) islands grown on the Cu(100) substrate forming the FeO/Cu2O/Cu(100) model surface and oxygen-covered Cu(100) (Cu2O/Cu(100)) have been prepared and characterized by scanning tunneling microscopy (STM), synchrotron radiation photoemission spectroscopy (SRPES), and low-energy electron diffraction (LEED). Annealing the FeO/Cu2O/Cu(100) and Cu2O/Cu(100) surfaces shows distinct differences in thermal stability. As compared to the pure Cu2O/Cu(100) surface, both STM and SRPES results indicate that the presence of FeO islands promotes the reduction of the oxygen-covered copper surface upon annealing. The absence of O2 signals in temperature-programmed desorption (TPD) experiments suggests that the oxygen adatoms diffuse into the copper bulk upon annealing up to 800 K and may react with segregated carbon atoms from bulk at temperatures higher than 800 K. Combining all these results, we conclude that the interface plays an important role in the properties of hybrid surface structures, and the obtained FeO/Cu2O/Cu(100) and Cu2O/Cu(100) surfaces may be used as model systems for studying the Cu–Fe-based catalysts.
Published as part of The Journal of Physical Chemistry virtual special issue “Energy and Catalysis in China”
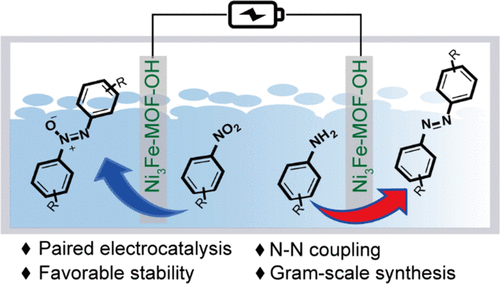
“Paired Electrochemical N−N Coupling Employing a Surface Hydroxylated Ni3Fe-MOF-OH Bifunctional Electrocatalyst with Enhanced Adsorption of Nitroarenes and Anilines”
Wei Qiao, Iqbal Waseem, Guangming Shang, Dan Wang, Yongwang Li, Flemming Besenbacher, Hans Niemantsverdriet, Chenglin Yan, and Ren Su, ACS Catalysis 11 (2021) 13510–13518
Abstract
Paired electrolysis employing both anodic and cathodic half reactions for the synthesis of value-added chemicals is an ultimate energy-efficient approach. Here, we show that paired reductive coupling of nitroaromatics into azoxy-aromatics and oxidative coupling of aromatic amines into azo-aromatics can be realized with high efficiency and selectivity employing the surfacehydroxylated Ni3Fe metal−organic framework (Ni3Fe-MOF-OH) bifunctional electrocatalyst. The competitive hydrogen and oxygen evolution reactions are suppressed due to the adsorption of nitroarenes and anilines via surface hydroxyls of the electrocatalyst. Simultaneous cathodic and anodic N−N coupling of a wide range of nitroaromatics and aniline derivatives are realized with high conversion and selectivity at an overall bias of 1.4 V in an undivided cell in 1 M KOH electrolyte. Ni3Fe-MOF-OH displays a high stability and enables gram-scale synthesis of azo- and azoxy-aromatics with a satisfactory yield and Faraday efficiency, offering an efficient synthetic protocol for applications.
“Carbon monoxide adsorption on cobalt overlayers on a Si(1 1 1) surface studied by STM and XPS”
Yang He, C.J. Weststrate, Dan Luo, J.W. Niemantsverdriet, Kai Wu, Jian Xu, Yong Yang, Yongwang Li, Xiaodong Wen, Appl. Surf. Sci. 569 (2021) 151045
Abstract
We report on the structure and reactivity of the Co-Si polycrystalline surfaces that form at room temperature (RT). Scanning tunneling microscopy (STM) was used to examine the surface morphology while X-ray photoelectron spectroscopy (XPS) was used to detect the chemical states of cobalt and silicon as a function of cobalt coverage. Moreover, XPS measurements after exposing the Co-Si(1 1 1) samples to CO gas provide information about CO adsorption and dissociation. When <5 ML Co is deposited on Si(1 1 1) at RT, most Co atoms diffuse into Si lattice and no CO adsorption is found at RT. This shows that coordination of Co to Si suppresses CO adsorption. Neither Co-induced Si(1 1 1)-ring cluster nor CoSi2 crystalline film adsorb CO at RT. In contrast, CO adsorption was found at RT for cobalt doses >5 ML, an indication that Co-rich silicides and metallic Co present. The CO adsorption capacity increases with cobalt dose up to 30 ML after which it levels off, an indication that metallic cobalt sites are dominantly exposed on the surface. Furthermore, a closed metal film is formed for 200 ML dose. Some dissociation of CO was observed during heating of the CO-covered samples for Co doses ≥30 ML.
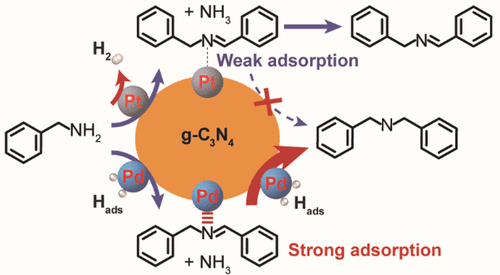
“Catalytic Role of Metal Nanoparticles in Selectivity Control over Photodehydrogenative Coupling of Primary Amines to Imines and Secondary Amines”
J. Yu, Q. Liu, W. Qiao, D. Lv, Y. Li, C. Liu, Y. Yu, Y. (Yongwang) Li, J.W. (Hans) Niemantsverdriet, B. Zhang, R. Su, ACS Catal. 11 (2021) 6656–6661
Abstract
Metal nanoparticles (NPs) are heavily involved in photocatalytic transformations to manipulate charge separation and storage, yet the catalytic role of metal NPs in tuning the selectivity of photoreactions is rarely addressed. Here, the photodehydrogenative coupling of primary amines is selected as the model reaction to probe the catalytic role of Pt and Pd NPs supported on graphitic carbon nitride (Pt/C3N4 and Pd/C3N4). When Pt/C3N4 is employed as the photocatalyst, imine is produced via dehydrogenative homocoupling of primary amines owing to the weak adsorption of photogenerated imines and H atoms on Pt NPs. In comparison, Pd/C3N4 promotes the consecutive hydrogenation of photogenerated imines into secondary amines due to a strong affinity of both imine and H atom for the surface of Pd NPs. This strategy is applicable for the synthesis of a series of imines and secondary amines with high yields.
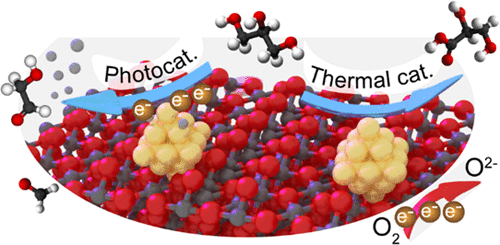
“Promotion Mechanisms of Au Supported on TiO2 in Thermal- and Photocatalytic Glycerol Conversion”
Y. Shen, A. Mamakhel, X. Liu, T. W. Hansen, T. Tabanelli, D. Bonincontro, B. B. Iversen, L. Prati, Y. (Yongwang) Li, J. W. (Hans) Niemantsverdriet, G. Hutchings, N. Dimitratos, A. Villa, R. Su, J. Phys. Chem. C 123 (2019) 19734-19741
Abstract
Catalytic glycerol conversion by means of either photon or thermal energy is of great importance and can be realized by metal supported on TiO2 systems. Although various procedures have been employed to synthesize efficient metal/TiO2 catalysts, the promotional mechanisms for both reactions are still unclear due to the lack of well-defined systems. Here, we have deposited gold nanoparticles on a series of highly crystalline anatase TiO2 substrates with different crystallite sizes (7, 12, 16, 28 nm) by both direct precipitation and sol-immobilization methods to examine the effect of metal deposition methods and TiO2 sizes on both photo- and thermal catalytic glycerol reforming. For photocatalytic H2 evolution from glycerol, optimum performance was observed for the Au supported on 12 nm TiO2 for both deposition methods. For thermal catalytic glycerol oxidation, all catalysts show a similar selectivity to glycerate (>70%) regardless of the TiO2 size and metal deposition method; however, the metal deposition method significantly influences the catalytic activity. In situ UV–vis spectrometry reveals that the optimized photocatalytic performance originates from enhanced charge transfer kinetics and a more negative Fermi level for proton reduction, whereas electrochemical analysis reveals that the promoted glycerol oxidation is caused by the enhanced oxygen reduction half-reaction.
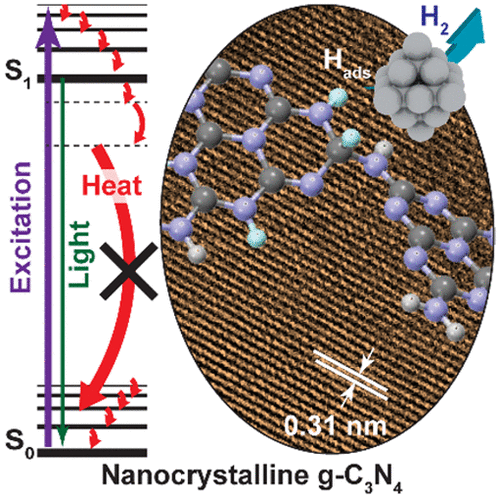
“Boosting Photocatalytic Hydrogen Production by Modulating Recombination Modes and Proton Adsorption Energy”
Y. Dai, Q. Bu, R. Sooriyagoda, P. Tavadze, O. Pavlic, T. Lim, Y. Shen, A. Mamakhel, X. Wang, Y. (Yongwang) Li, J.W. (Hans) Niemantsverdriet, B. B. Iversen, F. Besenbacher, T. Xie, J. P. Lewis, A. D. Bristow, N. Lock, R. Su, J. Phys. Chem. Lett. 10 (2019) 5381-5386
Abstract
PSolar-driven production of renewable energy (e.g., H2) has been investigated for decades. To date, the applications are limited by low efficiency due to rapid charge recombination (both radiative and nonradiative modes) and slow reaction rates. Tremendous efforts have been focused on reducing the radiative recombination and enhancing the interfacial charge transfer by engineering the geometric and electronic structure of the photocatalysts. However, fine-tuning of nonradiative recombination processes and optimization of target reaction paths still lack effective control. Here we show that minimizing the nonradiative relaxation and the adsorption energy of photogenerated surface-adsorbed hydrogen atoms are essential to achieve a longer lifetime of the charge carriers and a faster reaction rate, respectively. Such control results in a 16-fold enhancement in photocatalytic H2 evolution and a 15-fold increase in photocurrent of the crystalline g-C3N4 compared to that of the amorphous g-C3N4.
“Atomically Defined Iron Carbide Surface for Fischer-Tropsch Synthesis Catalysis: A molecular level picture and the effect of metal nanoparticles”
Y. Li, Z. Li, A. Ahsen, L. Lammich, G. J. A. Mannie, J. W. (Hans) Niemantsverdriet, J. V. Lauritsen, ACS Catal. 9 (2019) 1264-1273
Abstract
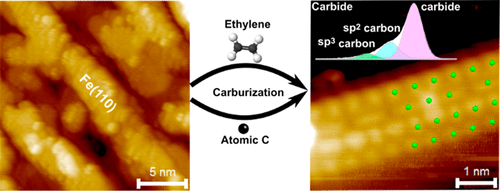
With the purpose of investigating the reactivity of Fe carbide as an active phase in Fischer–Tropsch catalysis, we studied the formation of a well-defined Fe carbide surface structure resulting from carbon exposure of an Fe film on Au(111). Using two different sources of carbon (C), namely atomic carbon and ethylene gas, we used synchrotron X-ray photoelectron spectroscopy (XPS) to show that a 6 ML Fe film readily converts into a well-defined and thermodynamically stable carbide phase. Scanning tunneling microscopy (STM) showed that the surface of the Fe carbide film is crystalline and is dominated by Fe(110)-like facets perturbed into a (2 × 2) periodic structure due to insertion of C in the interstitial sites. The reactivity of the carbide film toward CO, H2, and O2 was furthermore probed by XPS under vacuum conditions. While the pristine Fe carbide surface was unreactive toward hydrogen gas at 500 K, we interestingly found that CO dissociation from a preadsorbed monolayer of CO takes place already at low temperature. This observation points to an intrinsic activity of the Fe carbide phase where additional carbon originating from CO can be placed in the Fe carbide surface. The catalytic significance of the model catalyst surface presented here is that it can be seen as a stable Fe carbide phase with intrinsically vacant sites for additional C insertion at elevated pressure, and we propose that such additional C may act as active species in C–C coupling reactions during FTS. The studies pave the way for a better understanding of FTS processes on Fe-based catalysts on the basis of a well-defined model surface.
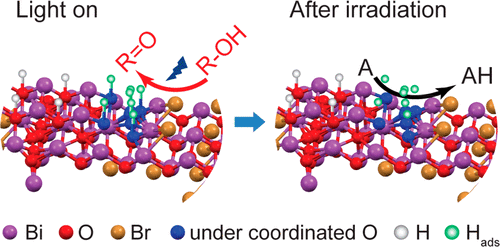
“Solid Base Bi24O31Br10(OH)δ with Active Lattice Oxygen for the Efficient Photo‐Oxidation of Primary Alcohols to Aldehydes”
Y. Dai, P. Ren, Y. Li, D. Lv, Y. Shen, Y. Li, J. W. (Hans) Niemantsverdriet, F. Besenbacher, H. Xiang, W. Hao, N. Lock, X. Wen, J. P. Lewis, R. Su, Angew. Chem. Int. Ed. 58 (2019) 6265-6270
Abstract
The selective oxidation of primary alcohols to aldehydes by O2 instead of stoichiometric oxidants (for example, MnVII, CrVI, and OsIV) is an important but challenging process. Most heterogeneous catalytic systems (thermal and photocatalysis) require noble metals or harsh reaction conditions. Here we show that the Bi24O31Br10(OH)δ photocatalyst is very efficient in the selective oxidation of a series of aliphatic (carbon chain from C1 to C10) and aromatic alcohols to their corresponding aldehydes/ketones under visible‐light irradiation in air at room temperature, which would be challenging for conventional thermal and light‐driven processes. High quantum efficiencies (71 % and 55 % under 410 and 450 nm irradiation) are reached in a representative reaction, the oxidation of isopropanol. We propose that the outstanding performance of the Bi24O31Br10(OH)δ photocatalyst is associated with basic surface sites and active lattice oxygen that boost the dehydrogenation step in the photo‐oxidation of alcohols.
“In-situ probing photocatalytic C-C bond cleavage in ethylene glycol under ambient conditions and the effect of metal cocatalyst”
C. Li, X. Wang, A. Cheruvathur, Y. Shen, H. Xiang, Y. (Yongwang) Li, J.W. (Hans) Niemantsverdriet, R. Su, J. Catal. 365 (2018) 313-319
Abstract
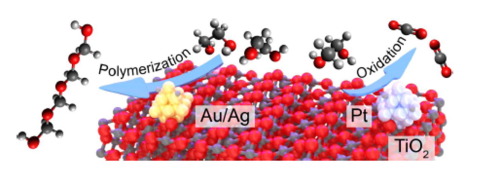
Photocatalytic polyol conversion provides a green approach for the synthesis of value-added products. However, efficient and selective photocatalysts that can prevent unwanted full oxidation are still missing, mostly due to a lack of mechanistic understanding. Here we use ethylene glycol (EG) as model compound to study the reaction pathways in photocatalytic polyol dissociation under aerated conditions using in–situ vibrational spectroscopy coupled with mass spectrometry. On pristine TiO2, the presence of oxygen leads to the formation of formaldehyde via photocatalytic C_C bond cleavage, where the removal of photo-generated surface adsorbed proton (Hads+) in the form of water is the rate determining step (RDS). The photo-generated formaldehyde molecules subsequently convert into CO2 via complete oxidation by oxygen, or into paraformaldehyde by polymerization with water. A promotion effect is observed when noble metal (Au, Pt, Ag) nanoparticles (NPs) are used as cocatalysts. While Ag and Au selectively promote the formation of paraformaldehyde, the addition of Pt facilitates the complete oxidation of EG into CO2. By performing the reaction under a low oxygen partial pressure, we rationalize that Ag and Au NPs accelerate the polymerization of formaldehyde by providing water rapidly through direct oxidation of Hads oxidation, whereas Pt NPs supply water indirectly, in a pathway via H2 or formaldehyde oxidation.
“Intercalation Mechanisms of Fe Atoms underneath A Graphene Monolayer on Ru(0001)”
Zhao, P. Ren, C. J. (Kees-Jan) Weststrate, Y. Xu, D. Cao, H. Xiang, J. Xu, Y. Yang, Y. (Yongwang) Li, J. W. (Hans) Niemantsverdriet, X. Wen, X. Yu, J. Phys. Chem. C 122 (2018) 22903-22910
Abstract
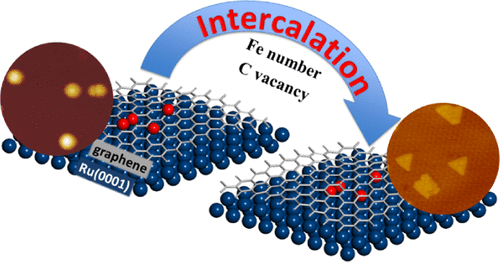
The intercalation process of iron atoms in the interface between graphene and Ru(0001) was systematically investigated both experimentally and computationally. Scanning tunneling microscopy and low-energy electron diffraction indicate that Fe intercalates at 700 K in the graphene/Ru(0001) system, where the graphene monolayer covers the whole substrate. An atomic-level understanding of the process is achieved using dispersion-corrected density functional theory (DFT) calculation. The results indicate that single-Fe atom intercalation causes only minor energy changes in the system. In contrast, the intercalation of a Fe dimer leads to a considerable drop in the total energy, more than twice the energy change in the case of the single-atom intercalation. In a sequential process, intercalation of the second Fe releases more energy, indicating that once the initial intercalation occurs, the subsequent process is thermodynamically more favored than the first. Combining the experimental observations with theoretical insights from the DFT calculations, we provide a clear picture of Fe intercalation into graphene/Ru(0001), which we believe is of interest to the field of interface and materials science and catalysis.
“Iron Carbidization on Thin-Film Silica and Silicon: A Near-Ambient-Pressure X-ray Photoelectron Spectroscopy and Scanning Tunneling Microscopy Study”
X. Zhou, G. J. A. Mannie, J. Yin, X. Yu, C. J. Weststrate, X. Wen, K. Wu, Y. Yang, Y. (Yongwang) Li, J. W. (Hans) Niemantsverdriet, ACS Catal. 8 (2018) 7326-7333
Abstract
Model catalysts consisting of iron particles with similar size deposited on thin-film silica (Fe/SiO2) and on silicon (Fe/Si) were used to study iron carbidization in a CO atmosphere using in situ near-ambient-pressure X-ray photoelectron spectroscopy. Significant differences were observed for CO adsorption, CO dissociation, and iron carbidization when the support was changed from thin-film silica to silicon. Stronger adsorption of CO on Fe/Si than that on Fe/SiO2 was evident from the higher CO equilibrium coverage found at a given temperature in the presence of 1 mbar of CO gas. On thin-film silica, iron starts to carbidize at 150 °C, while the onset of carbidization is at 100 °C on the silicon support. The main reason for the different onset temperature for carbidization is the efficiency of removal of oxygen species after CO dissociation. On thin-film silica, oxygen species formed by CO dissociation block the iron surface until ∼150 °C, when CO2 formation removes surface oxygen. Instead, on the silicon support, oxygen species readily spill over to the silicon. As a consequence, oxygen removal is not rate-limiting anymore and carbidization of iron can proceed at a lower temperature.
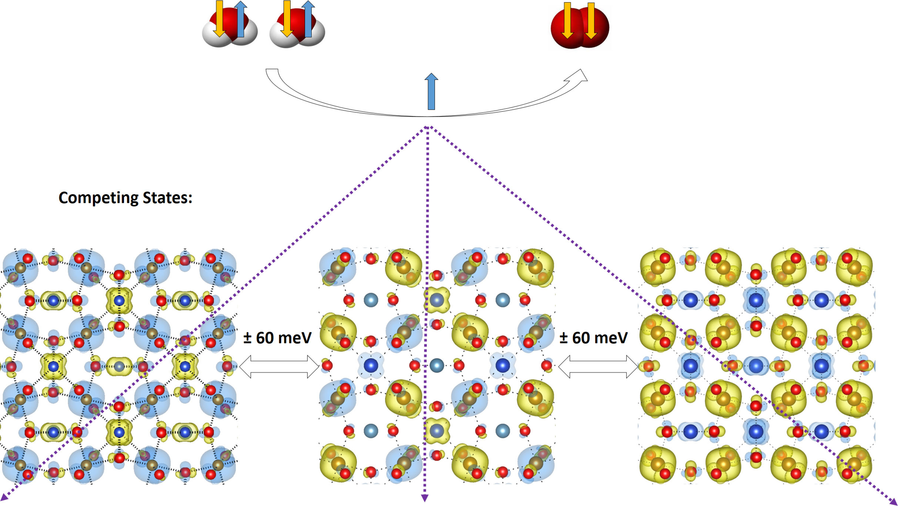
“Orbital Physics of Perovskites for the Oxygen Evolution Reaction”
R. Sharpe, J. Munarriz, T. Lim, Y. Jiao, J. W. (Hans) Niemantsverdriet, V. Polo, J. Gracia, Topics in Catalysis 61 (2018) 267–275
Abstract
The study of magnetic perovskite oxides has led to novel and very active compounds for O2 generation and other energy applications. Focusing on three different case studies, we summarise the bulk electronic and magnetic properties that initially serve to classify active perovskite catalysts for the oxygen evolution reaction (OER). Ab-initio calculations centred on the orbital physics of the electrons in the d-shell provide a unique insight into the complex interplay between spin dependent interactions versus selectivity and OER reactivity that occurs in these transition-metal oxides. We analyse how the spin, orbital and lattice degrees of freedom establish rational design principles for OER. We observe that itinerant magnetism serves as an indicator for highly active oxygen electro-catalysts. Optimum active sites individually have a net magnetic moment, giving rise to exchange interactions which are collectively ferromagnetic, indicative of spin dependent transport. In particular, optimum active sites for OER need to possess sufficient empty orthogonal orbitals, oriented towards the ligands, to preserve an incoming spin aligned electron flow. Calculations from first principles open up the possibility of anticipating materials with improved electro-catalytic properties, based on orbital engineering.
“Efficient Solar-Driven Hydrogen Transfer by Bismuth-Based Photocatalyst with Engineered Basic Sites”
Y. Dai, C. Li, Y. Shen, S. Zhu, M. S. Hvid, L. Wu, J. Skibsted, Y. (Yongwang) Li, J. W. (Hans) Niemantsverdriet, F. Besenbacher, N. Lock, R. Su, JACS 140 (2018) 16711-16719
Abstract
Photocatalytic organic conversions involving a hydrogen transfer (HT) step have attracted much attention, but the efficiency and selectivity under visible light irradiation still needs to be significantly enhanced. Here we have developed a noble metal-free, basic-site engineered bismuth oxybromide [Bi24O31Br10(OH)δ] that can accelerate the photocatalytic HT step in both reduction and oxidation reactions, i.e., nitrobenzene to azo/azoxybenzene, quinones to quinols, thiones to thiols, and alcohols to ketones under visible light irradiation and ambient conditions. Remarkably, quantum efficiencies of 42% and 32% for the nitrobenzene reduction can be reached under 410 and 450 nm irradiation, respectively. The Bi24O31Br10(OH)δ photocatalyst also exhibits excellent performance in up-scaling and stability under visible light and even solar irradiation, revealing economic potential for industrial applications.
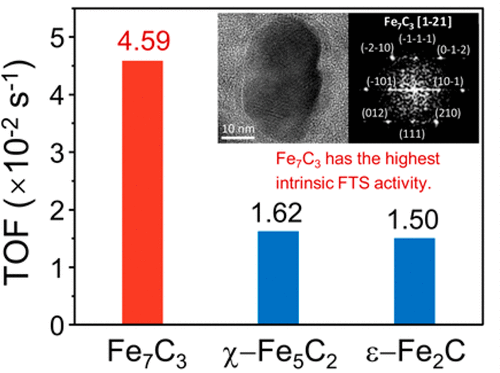
“Relationship between Iron Carbide Phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and Catalytic Performances of Fe/SiO2 Fischer–Tropsch Catalysts”
Q. Chang, C. Zhang, C. Liu, Y. Wei, A. V. Cheruvathur, A. I. Dugulan, J. W. (Hans) Niemantsverdriet, X. Liu, Y. He, M. Qing, L. Zheng, Y. Yun, Y. Yang. Y. (Yongwang) Li, ACS Catal. 8 (2018) 3304-3316
Abstract
The influence of different iron carbides on the activity and selectivity of iron-based Fischer–Tropsch catalysts has been studied. Different iron carbide phases are obtained by the pretreatment of a binary Fe/SiO2 model catalyst (prepared by coprecipitation method) to different gas atmospheres (syngas, CO, or H2). The phase structures, compositions, and particle sizes of the catalysts are characterized systematically by XRD, XAFS, MES, and TEM. It is found that in the syngas-treated catalyst only χ-Fe5C2 carbide is formed. In the CO-treated catalyst, Fe7C3 and χ-Fe5C2 with a bimodal particle size distribution are formed, while the H2-treated catalyst exhibits the bimodal size distributed ε-Fe2C and χ-Fe5C2 after a Fischer–Tropsch synthesis (FTS) reaction. The intrinsic FTS activity is calculated and assigned to each corresponding iron carbide based on the phase composition and the particle size. It is identified that Fe7C3 has the highest intrinsic activity (TOF = 4.59 × 10–2 s–1) among the three candidate carbides (ε-Fe2C, Fe7C3, and χ-Fe5C2) in typical medium-temperature Fischer–Tropsch (MTFT) conditions (260–300 °C, 2–3 MPa, and H2/CO = 2). Moreover, FTS over ε-Fe2C leads to the lowest methane selectivity.
“Light-tuned selective photosynthesis of azo- and azoxy-aromatics using graphitic C3N4“
Yitao Dai, Chao Li, Yanbin Shen, Jian Xu, Hans Niemantsverdriet, Yongwang Li, Flemming Besenbacher, Nina Lock, Ren Su, Nature Communications 9 (2018) Article number 60
(Open access)
Abstract
Solar-driven photocatalysis has attracted significant attention in water splitting, CO2 reduction and organic synthesis. The syntheses of valuable azo- and azoxyaromatic dyes via selective photoreduction of nitroaromatic compounds have been realised using supported plasmonic metal nanoparticles at elevated temperatures (≥90 °C); however, the high cost, low efficiency and poor selectivity of such catalyst systems at room temperature limit their application. Here we demonstrate that the inexpensive graphitic C3N4 is an efficient photocatalyst for selective syntheses of a series of azo- and azoxy-aromatic compounds from their corresponding nitroaromatics under either purple (410 nm) or blue light (450 nm) excitation. The high efficiency and high selectivity towards azo- and azoxy-aromatic compounds can be attributed to the weakly bound photogenerated surface adsorbed H-atoms and a favourable N-N coupling reaction. The results reveal financial and environmental potential of photocatalysis for mass production of valuable chemicals.
“Photocatalytic C-C bond cleavage in ethylene glycol on TiO2: A molecular level picture and the effect of metal nanoparticles”
Xianchi Jin, Chao Li, Chenbiao Xu, Dawei Guan, Ajin Cheruvathur, Yi Wang, Jian Xu, Dong Wei, Hongwei Xiang, J.W. (Hans) Niemantsverdriet, Yongwang Li, Qing Guo, Zhibo Ma, Ren Su, Xueming Yang, J. Catal. 354 (2017) 37–45
Abstract
Polyol conversion to value-added products is of great interest for the bio-diesel industry. Photocatalytic oxidation processes may offer a green approach for polyol conversion; however the lack of comprehensive mechanistic understanding from an interdisciplinary perspective limits or even misleads the design of highly selective and efficient photocatalysts for such process. Here we have studied the photocatalytic polyol conversion on pristine TiO2 and metal (Au, Pd, and Pt) nanoparticles (NPs) decorated TiO2 using ethylene glycol (EG) as the model compound. We have developed a mechanistic picture at molecular level by coupling in-situ surface science study on rutile (110) surface with in-situ vibrational-mass spectrometry study on TiO2 nanopowders. The C-C bond cleavage was found to be the only pathway in EG photo-conversion under deaerated conditions, leading to the formation of formaldehyde and hydrogen. We rationalized that the desorption of the surface adsorbed H (Hads) to be the rate determining step (RDS), making pristine TiO2 a poor photocatalyst that only catalyze the EG conversion at very low surface coverages. The addition of metal NPs on TiO2 surface promotes the desorption of Hads significantly, thus leading to an enhanced C-C bond cleavage performance at higher surface coverages that is more applicable.
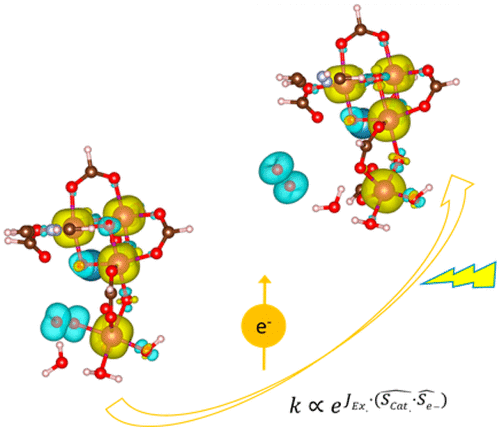
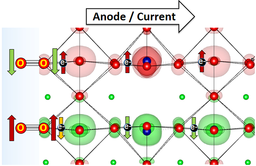
“Analysis of the Magnetic Entropy in Oxygen Reduction Reactions Catalysed by Manganite Perovskites”
Jose Gracia, Julen Munarriz, Victor Polo, Ryan Sharpe, Yunzhe Jiao, Hans Niemantsverdriet, Tingbin Lim, ChemCatChem 9 (2017) 3358-3363
Abstract
Manganese oxides with a half‐metallic ground state are particularly active for oxygen reduction reactions (ORR). La0.67Sr0.33MnO3 (LSMO) perovskite is the archetypal example for compositions with a Curie temperature (TC) above room temperature and with a high intrinsic activity for the partial reduction of triplet‐state O2. The ferromagnetic (FM) character of the superexchange interactions in LSMO facilitates both charge and spin transport below 370 K. Other than the enhanced electronic conductivity, the reduced spin entropy seems to be relevant in oxygen catalysis because the magnetic ordering extends to the surface. The sign of the exchange interactions determines the adsorption of the triplet oxygen molecule with its spin antiparallel to the FM catalysts. Based on the transition‐state theory, we report that on LSMO, the hindrance resulting from the magnetic entropy for the initial reduction of O2 by two antiparallel electrons to diamagnetic intermediates (such H2O2) is minimum. On the other hand, the additional reduction of H2O2 to H2O, diamagnetic steps, prefers paramagnetic catalysts with higher magnetic entropy such as La0.4Sr0.6MnO3 to avoid spin accumulation.
“Photosystem II Acts as a Spin-Controlled Electron Gate during Oxygen Formation and Evolution”
Yunzhe Jiao, Ryan Sharpe, Tingbin Lim, Hans Niemantsverdriet, Jose Gracia, JACS 139 (2017) 16604–16608
Abstract
The oxygen evolution complex (OEC) of photosystem II (PSII) is intrinsically more active than any synthetic alternative for the oxygen evolution reaction (OER). A crucial question to solve for the progress of artificial photosynthesis is to understand the influential interactions during water oxidation in PSII. We study the principles of interatomic electron transfer steps in OER, with emphasis on exchange interactions, revealing the influence of delocalizing ferromagnetic spin potentials during the catalytic process. The OEC is found to be an exchange coupled mixed-valence electron-spin acceptor where its orbital physics determine the unique activity of PSII. The two unpaired electrons needed in the triplet O2 molecule interact with the high spin state of the catalyst via exchange interactions; the optimal ferromagnetic catalyst and the resulting radical intermediates are spin paired. As a result, the active center of the CaMn4O5 cofactor, stimulated by the driving potential provided by photons, works as a spin valve to accelerate the formation and release of O2 from diamagnetic H2O.
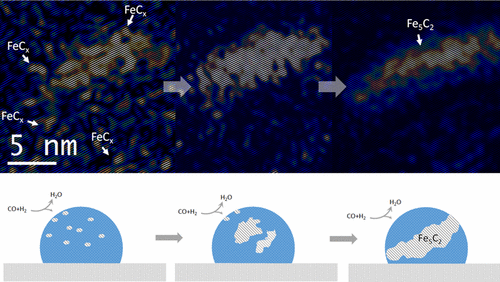
“Environmental Transmission Electron Microscopy (ETEM) Studies of Single Iron Nanoparticle Carburization in Synthesis Gas”
Xi Liu, Chenghua Zhang, Yongwang Li, J. W. Niemantsverdriet, Jakob B. Wagner, and Thomas W. Hansen, ACS Catal. 7 (2017) 4867−4875
Abstract
Structural evolution of iron nanoparticles involving the formation and growth of iron carbide nuclei in the iron nanoparticle was directly visualized at the atomic level, using environmental transmission electron microscopy (TEM) under reactive conditions mimicking Fischer−Tropsch synthesis. Formation of the iron carbide nuclei and surface reconstruction of the iron nanoparticle play an essential role in carburization of the iron nanoparticle and consequent formation of Fe5C2. Identification of carbide and oxide intermediates evidenced by high-resolution TEM images, electron diffraction patterns and electron energy-loss spectra provides a detailed picture from initial activation to final degradation of iron under synthesis gas.
“Graphene with Atomic-Level In-Plane Decoration of h-BN Domains for Efficient Photocatalysis”
Mengzhu Li, Yiou Wang, Pei Tang, Nanhong Xie, Yunxuan Zhao, Xi Liu, Gang Hu, Jinglin Xie, Yufei Zhao, Junwang Tang, Tierui Zhang, and Ding Ma, Chem. Mater. 29 (2017) 2769–2776
Abstract
Band gap opening and engineering make up one of the primary goals of developing novel materials for photocatalytic hydrogen generation. We report here a facile synthesis of graphene decorated with in-plane boron nitride domains via control of both the doping sequence of heteroatoms and the oxygen content of the graphene precursor, showing significant differences in the doping pattern compared with B and/or N singly doped or co-doped graphene. We uncover that the formation of BN domains in graphene is critical for engineering the band gap and delivering an improved activity for photocatalytic hydrogen generation in the absence of any photosensitizer. This work paves the way for the rational design and construction of graphene-based photocatalysts for efficient photocatalysis.
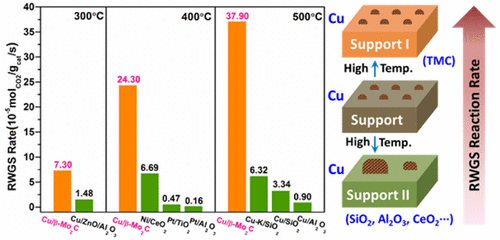
“Highly Dispersed Copper over β-Mo2C as an Efficient and Stable Catalyst for the Reverse Water Gas Shift (RWGS) Reaction”
Xiao Zhang, Xiaobing Zhu, Lili Lin, Siyu Yao, Mengtao Zhang, Xi Liu, Xiaoping Wang, Yong-Wang Li, Chuan Shi, and Ding Ma, ACS Catal. 7 (2017) 912–918
Abstract
Cu-oxide catalysts have a tendency to deactivate dramatically in reverse water gas shift (RWGS) reaction, because of the aggregation of supported copper particles at high temperatures. Herein, β-Mo2C, which is a typical type of transition-metal carbide, has been demonstrated to be capable of dispersing and stabilizing copper particles. Cu/β-Mo2C catalysts exhibit good catalytic activity and stability for the RWGS reaction. Under relatively high weight hourly space velocity (WHSV = 300 000 mL/g/h), the optimized 1 wt % Cu/β-Mo2C exhibits superior activity over traditional oxide-supported Pt- and Cu-based catalysts. The activity was well-maintained in a 40 h stability test, and the catalyst shows stable reactivity in a six-cycle start-up cool-down experiment. Detailed structure characterizations demonstrate that the strong interaction between Cu and β-Mo2C effectively promotes the dispersion of supported copper and prevents the aggregation of Cu particles, which accounts for the extraordinary activity and stability for the RWGS reaction.
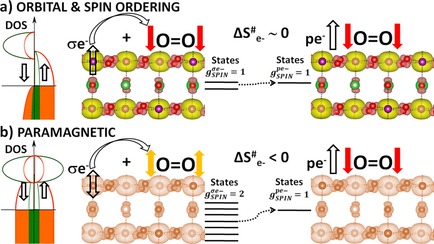
“Oxygen Evolution Reaction on Perovskite Electrocatalysts with Localized Spins and Orbital Rotation Symmetry”
Ryan Sharpe, Tingbin Lim, Yunzhe Jiao, J. W. (Hans) Niemantsverdriet, Jose Gracia, ChemCatChem 8 (2016) 37628-3768
Abstract
We have studied, by using ab initio calculations, the electronic properties of electro-catalysts for the oxygen evolution reaction (OER) with polarised density of states caused by localised spins in the d shell. Oxygen is a molecule in the triplet state (i.e. the outer electrons have parallel spins), which means that the spins localised in the p shell (↑O=O↑), the d shell and the conduction band electrons (t2gnegm) will couple through exchange interactions, which we think will provide favourable conditions for the OER. We compare the perovskites CaCu3Fe4O12 (CCF) and Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) with RuO2. CCF and BSCF both have fluctuating electronic structures accessible at room temperature that are linked to conducting spin-polarised density of states, equivalent to the paramagnetic state of covalent transition metal oxides with fine charge conductivity. CCF and BSCF both possess a considerable number of unpaired electrons localised in the inner d shell, high-spin configurations and competing inter-atomic exchange interactions. As a first approximation, the average fluctuation of the magnetisation in the metal atoms correlates linearly with the OER onset potential for the studied compositions. By linking the dynamics of the localised inner-electron spins to the conduction spins through exchange interactions, we can predict that other perovskites, such as Sr2Fe0.75Co0.25MoO6, will be OER active at room temperature, as they have similar electronic properties to CCF and BSCF.
“Layered Antiferromagnetic Ordering in the Most Active Perovskite Catalysts for the Oxygen Evolution Reaction”
Tingbin Lim, J. W. (Hans) Niemantsverdriet, Jose Gracia, ChemCatChem 8 (2016) 2968–2974
Abstract
We have performed an in-depth ab initio study of the magnetic structure within the most active perovskites for the oxygen evolution reaction. In all cases, the ground state exhibits an extended antiferromagnetic coupling in the unit cell. Layered antiparallel alignment of the magnetic moments appears to be related to their electrocatalytic activity. All the perovskites calculated within this paper show space-separated charge-transport channels depending on the spin orientation. Comparing the electronic structures with the reported activities, we find a direct correlation between the magnetic accumulation on the spin channels in the bulk material and the catalytic activity. We discuss the possible implications of such observations in terms of magnetic interactions. During oxygen evolution in water electrolysis, reactants and products do not preserve spin. For triplet state oxygen to evolve, the catalyst at the anode can speed up the reaction if it is able to balance the magnetism of the oxygen molecule by extracting electrons with an opposite magnetic moment, conserving the overall spin.
“Mechanistic Insight into the Interaction Between a Titanium Dioxide Photocatalyst and Pd Co-catalyst for Improved Photocatalytic Performance”
Ren Su, Nikolaos Dimitratos, Jinjia Liu, Emma Carter, Sultan Althahban, Xueqin WANG, Yanbin Shen, Stefan Wendt, Xiaodong Wen, J. W. (Hans) Niemantsverdriet, Bo B. Iversen, Christopher J. Kiely, Graham J. Hutchings, and Flemming Besenbacher, ACS Catal. 6 (2016) 4239
Abstract
Understanding the co-catalyst/semiconductor interaction is of key importance for the design and synthesis of next generation photocatalytic materials for efficient hydrogen production and environmental clean-up applications. Here we investigate preformed Pd nanoparticles (NPs) supported on a series of anatase TiO2 having well-controlled but varying degrees of crystallinity and crystallite size, and explore their photocatalytic performance for H2 production and phenol decomposition. Whilst tuning the anatase crystallite size significantly influences the photocatalytic performance, varying the TiO2 crystallinity shows a negligible effect. Interestingly, the optimum quantum efficiency (~78%) for H2 evolution is achieved with anatase having medium crystallite size (~16 nm), whereas for phenol decomposition, a promotional effect is only observed for anatase with larger crystallite sizes (> 20 nm). Surface radical species and radical densities study reveal that the photogenerated charge carriers have been trapped at different sites depending on the crystallite size of anatase. Whilst the excited electrons are only trapped in bulk lattice sites in small anatase (<16 nm), larger anatase particles provide extra surface sites for charge trapping, which benefit charge storage and transportation to Pd surface sites, leading to a more efficient utilisation of charge carriers for photocatalysis. Additionally, Pd supported on medium-size anatase (~16 nm) hinders the formation of O2•- radicals on TiO2 surfaces, thus preventing unwanted re-oxidation of photogenerated H2.