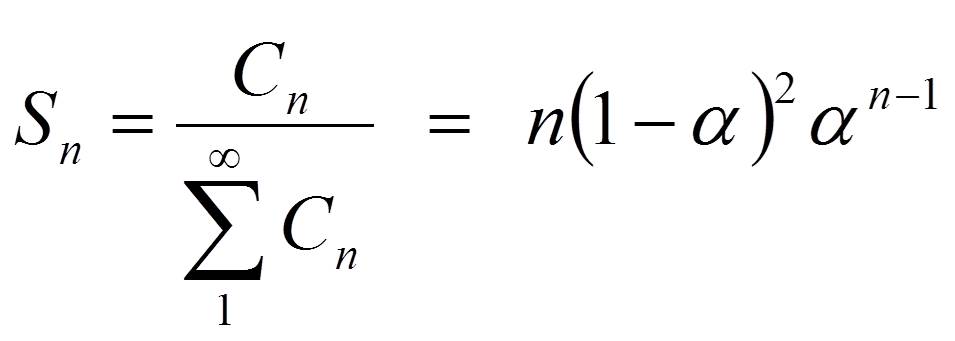Synthesis Gas Chemistry and Synthetic Fuels

Introduction
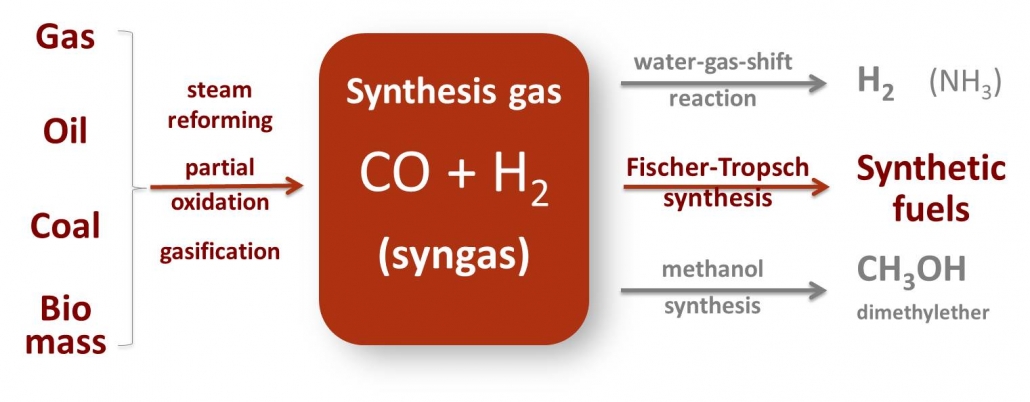
Synthesis gas or briefly, syngas, is a mixture of carbon monoxide, carbon dioxide and hydrogen. Syngas can be produced from many sources, including natural gas, coal, biomass, or virtually any hydrocarbon feedstock, by reaction with steam or oxygen. And nowadays also from CO2 and H2 obtained by electrolysis or by thermal composition of CO2 and H2O by concentrated solar light. Syngas is a crucial intermediate resource for production of hydrogen, ammonia, methanol, and synthetic hydrocarbon fuels.
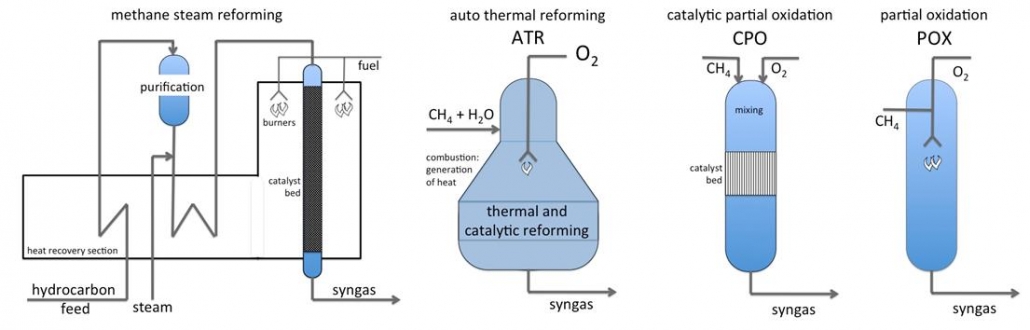
The formation of syngas is strongly endothermic and requires high temperatures. Steam reforming of natural gas (or shale gas) proceeds in tubular reactors that are heated externally. The process uses nickel catalyst on a special support that is resistant against the harsh process conditions. Waste heat from the oven section is used to preheat gases and to produce steam. This plant generates syngas with H2/CO ratios in the range of 3-4, and is suitable for hydrogen production.
Partial oxidation of methane (or hydrocarbons) is a non-catalytic, large-scale process to make syngas and yields syngas with H2/CO ratio of about 2. This is an optimal ratio for gas-to-liquids plants. A catalytic version of partial oxidation (CPO), based on short-contact time conversion of methane, hydrocarbons or biomass on e.g. rhodium catalysts, is suitable for small-scale applications.
Autothermal reforming (ATR) is a hybrid, which combines methane steam reforming and oxidation in one process. The heat needed for reforming is generated inside the reactor by oxididation of the feed gas. As POX, ATR is also suitable for large-scale production of syngas for gas-to-liquids or large-scale methanol synthesis processes.
Alternative routes to syngas, such as reduction of CO2 from flue gas with H2from electrolytic splitting of water may become interesting from the viewpoint of storage of wind or solar energy.
Scheme 1 – Synthesis gas (syngas) can be produced from a variety of sources and is a versatile intermediate for production of chemicals and fuels. Gas-to-Liquids (GTL), Coal-to-Liquids (CTL), Biomass-to-Liquids (BTL) all rely on the catalytic conversion of syngas.
Figure 1 – Reactors and process layout for syngas production from natural gas and shale gas.
Hydrogen from Syngas
In the water-gas-shift reaction, CO is used as a reductor to shift syngas entirely to H2 (and CO2):
CO + H2O ⇋ CO2 + H2 – 41 kJ/mol
The high temperature water gas shift uses iron oxide as a catalyst and proceeds at 300-500 °C. A low-temperature process (around 200 °C) based on a copper-zinc oxide catalyst drives the equilibrium further towards hydrogen, but requires clean feed gas. Pressure swing adsorption purification leads to high-purity hydrogen.
Syngas to Methanol
Methanol is a versatile intermediate for the chemical industry, but can also serve as a fuel. Even better is dimethyl ether, applicable as bottle gas for cooking (like camping gas) or as a substitute for diesel fuel. Methanol is also used in the transesterification of vegetable oils to produce biodiesel. Methanol is produced catalytically from a mixture CO2:CO:H2 = 5:5:90, at 50-100 bar and 225-275 °C over Cu/ZnO/Al2O3.
The predominant reactions are
CO2 + 3 H2 ⇋ CH3OH + H2O – 47 kJ/mol
or combined with the water-gas shift reaction,
CO + 2 H2 ⇋ CH3OH – 91 kJ/mol
Copper metal is the catalytically active phase, and ZnO is a chemical and structural promoter, while alumina is only a structural promoter.
Syngas to Synfuels –
The Fischer-Tropsch Synthesis
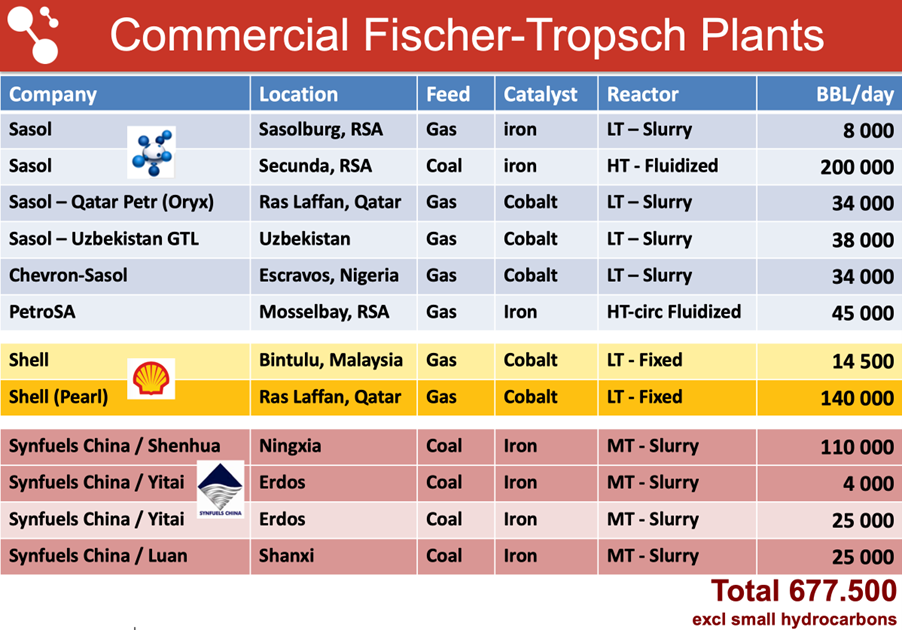
The Fischer-Tropsch Synthesis (FTS) converts syngas into hydrocarbons which form the basis for gasoline, diesel, jet fuel, and chemicals such as olefins and waxes. It forms the heart of the Gas-to-Liquids (GTL) and Coal-to-Liquids (CTL) plants in South Africa, Qatar, Nigeria, Uzbekhistan, Malaysia and China (see Table 1). The product distribution is broader than liquid hydrocarbons alone, and includes methane and alkanes, CnH2n+2 (with n from 1 → 100), alkenes or olefins (CnH2n; n ≥ 2), and to a lesser extent oxygenated products such as alcohols. Catalysts for the Fischer-Tropsch Synthesis are either based on cobalt or the much cheaper iron. In fact, the iron-based catalyst is an iron carbide under reaction conditions, whereas cobalt works in the metallic state.
The overall reaction equations are straightforward (but hide a tremendous amount of mechanistic complexity):
n CO + (2n+1) H2 → CnH2n+2 + n H2O
n CO + 2n H2 → CnH2n + n H2O
n CO + 2n H2 → CnH2n+1OH + (n-1) H2O
Reaction conditions include temperatures between 200 and 350 °C and pressures between 20 and 50 bar. The reactions are exothermic and dealing with the heat is an important issue in the reactor design.
Table 1 – Commercial gas and coal to liquids Fischer-Tropsch plants with a capacity of 4000 barrels per day and more in the world.
The reactions produce water, which is the predominant pathway to remove the O-atom from the CO. Iron catalysts have activity for water gas shift, implying that the formed product water my shift to H2 and CO2 making iron-based FTS less dependant on the initial H2:CO ratio, which may be as low as 0.5: just add the WGS reaction to the symbolic FTS reaction equation per one CO molecule:
CO + 2H2 = -CH2– + H2O
which gives:
2CO + H2 ⇋ -CH2– + CO2
This makes Fe-based FTS the preferred option for Coal-to-Liquids technology.
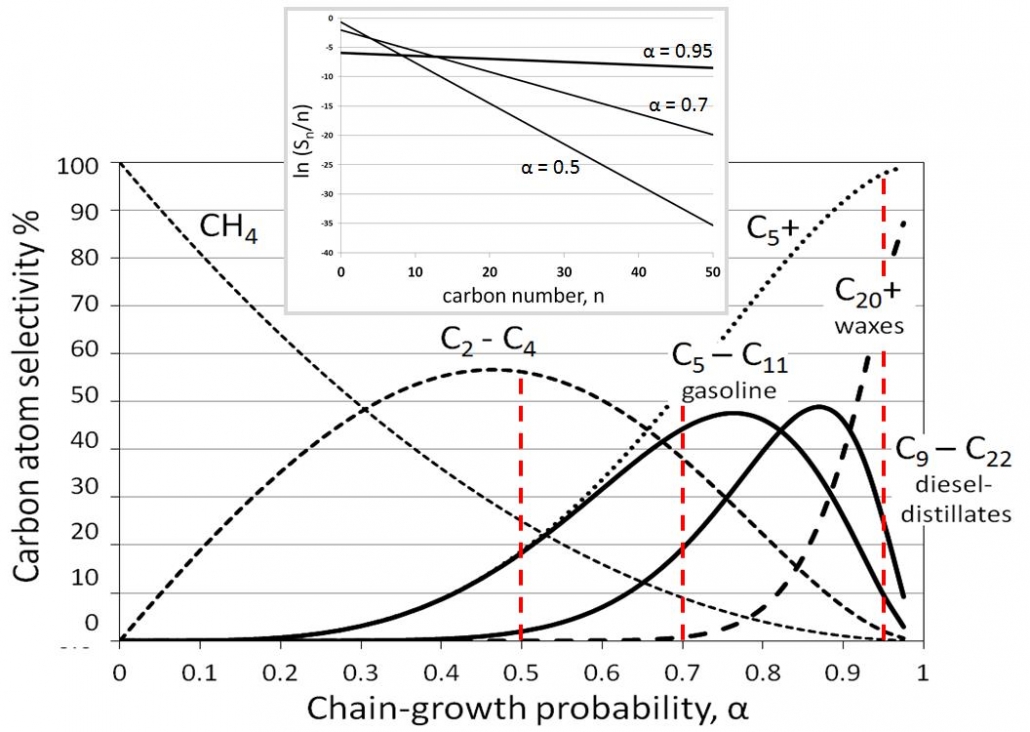
Figure 2 – Fischer-Tropsch product distributions in terms of interesting hydrocarbon fractions as a function of the chain-growth, alpha. The insert shows a few Anderson-Schulz-Flory plots according to the logarithmic form.
Hydrocarbon Product Distribution
Fischer-Tropsch synthesis is a polymerization process. Even without knowing the exact mechanism, we can express the selectivity towards a product with n carbon atoms as
where C stands for the concentration of a hydrocarbon with n carbon atoms and α is the probability that the chain grows.
This is the Anderson-Flory-Schultz distribution, see Figure 2, for how the Fischer-Tropsch product distribution depends on the chain growth probability, α. Converting it in logarithmic form provides an easy opportunity to verify if a measured selectivity pattern matches this distribution if one plots ln (Sn/n) versus n, see the inset in Figure 2.
The ASF distribution describes the product distribution reasonably well. One should not expect that the methane yield (usually higher) or the C2 yield (often lower than predicted) satisfy the ASF distribution, as these molecules do not form through these same polymerization mechanisms, and it is also common to observe a higher chain-growth probability for longer hydrocarbons than for shorter ones.
Fischer-Tropsch Technology
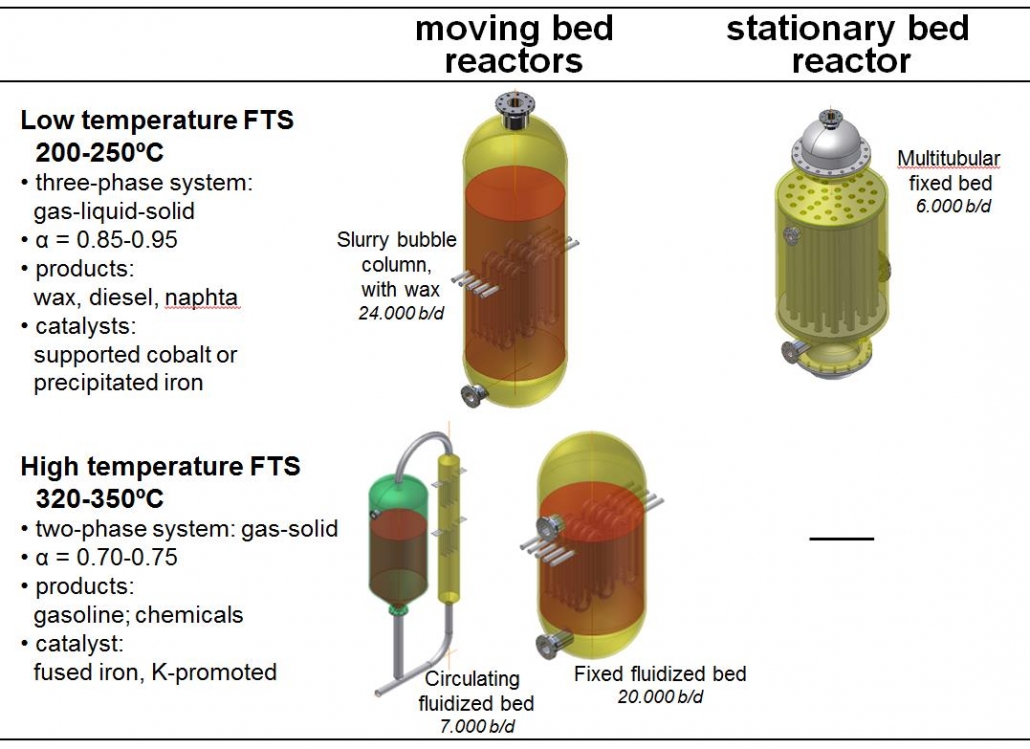
Figure 3 shows the different reactor types that are presently in use, along with process conditions. Low temperature FTS is good for long chains, waxes, and high-temperature for shorter products, i.e. if chemicals and/or gasoline are the desired product. LTFT reactors are three-phase systems, with solid catalysts, gaseous reactants, and gaseous and liquid products. LT and MTFT form the basis for the modern GTL and CTL plants. The wax is subsequently hydrocracked to form mostly diesel fuel and some naphta.
Fischer-Tropsch Catalysts
Both iron and cobalt catalysts can be used for the LTFT processes in CTL and GTL plants. Iron-based LTFT catalysts contain promoters like potassium and copper as well as a structural modifier such as silica. These catalysts are normally prepared by co-precipitation of the iron and copper elements, followed by impregnation with the potassium and structural promoters. Thereafter, the catalyst has to be shaped for use in either a fixed bed (e.g. extrudates) or a slurry phase FT reactor (e.g. spraydried spheres), followed by calcination. Prior to use in the FT process, the oxidized iron catalysts need to be activated or conditioned in hydrogen or synthesis gas. During the FT process the iron metal is converted to an iron carbide, and depending on the conditions, oxidized again to an iron oxide.
Figure 3 – Overview of Fischer-Tropsch technologies and reactor types used.
Outlook
Fischer-Tropsch synthesis represents proven technology for conversion of gas and coal into synthetic fuels, already since 90 years ago. It gained recently interest in the context of converting solar and wind electricity to synthetic fuels and chemicals (Power to X) as well. Although much scientific research has concentrated on GTL technology and cobalt catalysts, it is the CTL technology based on iron catalysts that recently has seen the largest growth, in China, although cobalt catalysts are often used in small scale FTS plants, e.g. for Power-to-Liquids applications. Significant improvements of the iron-based catalysts have been realized, but improvements in stability and control over selectivity are still welcome. In fact, scientific understanding of the complex iron-carbon-oxygen phases as they feature in the process, and the relation between catalyst composition and the surface chemistry has still not developed very far beyond insight in highly simplified systems. In this respect the metallic cobalt FTS catalyst is significantly better understood than its iron counterpart.
This text has mostly been based on the following literature:
- Rostrup-Nielsen, L.J. Christiansen, Concepts in Syngas Manufacture, Catalytic Science Series – Vol. 10, Imperial College Press, London, 2011
- van de Loosdrecht and J.W. Niemantsverdriet, Synthesis Gas to Hydrogen, Methanol and Synthetic Fuels, in “Chemical Energy Storage” (R. Schlögl, Ed.), De Gruyter, Berlin, 2013
- van de Loosdrecht, F.G. Botes, I.M. Ciobica, A. Ferreira, P. Gibson, D.J. Moodley, A.M. Saib, J.L. Visagie, C.J. Weststrate, J.W. Niemantsverdriet, Fischer–Tropsch synthesis: catalysts and chemistry, in Comprehensive Inorganic Chemistry II (Elsevier, Amsterdam, 2013), 525-557. doi:10.1016/B978-0-08-097774-4.00729-4
- J. Xu, Y. Yang, Y.-W. Li, Recent Developments in Converting Coal to Clean Fuels in China, Fuel 2015, 152, 122−130.
- M. Berger, M. Muhler, J. Van de Loosdrecht, J.W. Niemantsverdriet, E. Kunkes, K.F. Ortega, M. Behrens, CO2 uitilization: Methane, Methanol, And Synthetic Fuels, in “Chemical Energy Storage, 2nd Edition (R Schlögl, Ed.), De Gruyter, Berlin 2022.